
By using the fractional distillation technique, we can separate several gases from liquid air. It is composed of various concentrations of gases including nitrogen, oxygen, carbon dioxide, argon, and others.

At each distillation the vapour is richer in nitrogen (the component. Solution Fractional distillation: Air is a homogenous mixture of gases.
#Obtained from liquid air by fractional distillation free#
You are free to add your own answers below this page to assist others improve their learning.Miscible liquids are ones that can mix together – like water and ethanol. Nitrogen is gotten in large quantity from fractional distillation of liquid air.Gaseous mixtures like air are firstly liquified before distilling fractionally.Fractional distillation is usually done on liquids.Evaporation will rather be used to separate sulphur from carbon disulphide. this can be manipulated to form a boundary layer separable by separating funnel method. Evaporation will rather be appropriate here. Nitrogen is obtained from liquid air by fractional distillation. Now for the right answer to the above question: refers to equipment which obtain oxygen, nitrogen and argon from liquid air at low. RELATED => The process whereby some organisms with certain. Fractional Distillation of Nitrogen/ Liquid Oxygen Cylinder Filling.

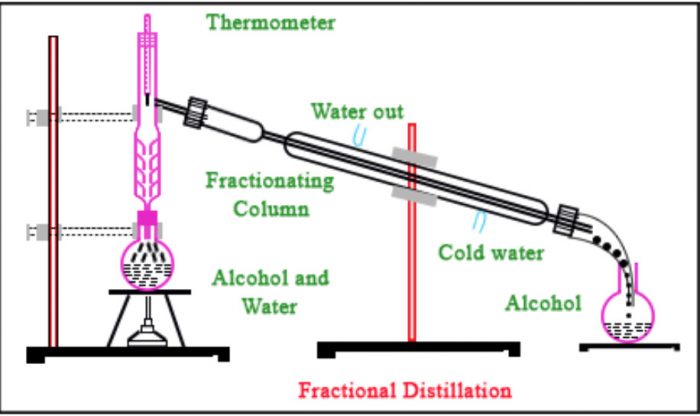
Air is firstly liquified by subjecting to intense pressure and reduced temperature, and then the resultant liquified air is subjected to fractional distillation to separate it into its components which are: Nitrogen, oxygen, carbon dioxide, and noble gasses. The key word here is liquids of different boiling points. Heat is usually applied to take advantage of the differences in boiling points of each of the components to isolate them beginning from the component with the lowest boiling point. Sulphur from the solution of sulphur in carbon disulphide QUICK ANSWER…įractional distillation is a separation technique used to separate two or more liquids of different boiling points. Iodine from solution of Iodine in carbon tetrachlorideĭ. The vapors condense on this cool surface, and the condensed liquid (called the 'distillate') drips into a reservoir separated from the original liquid. Which of the following can be obtained by fractional distillation?Ĭ. In a distillation, a liquid is boiled in the 'distilling flask,' then the vapors travel to another section of the apparatus where they come into contact with a cool surface. In the diagram above, a bulb is lit by drawing 2.0A from 440V a.c. Fractional distillation is a separation method where the difference in boiling points of components is used to separate the liquid mixture into fractions.Find the simple interest on N225 in 5 years at 10%….


 0 kommentar(er)
0 kommentar(er)
